ABOUT FABRY DISEASE
Fabry disease worsens over time and can become life-threatening. It is important to talk to your doctor and understand how Fabry disease may affect your body.
DISEASE OVERVIEW
What is Fabry disease?
Fabry disease is an inherited condition caused by a genetic variation, a change in one of your genes. Because of this change, your body is unable to make enough of an enzyme called alpha-galactosidase A, or alpha-GAL. Enzymes are proteins that break down substances in your body. When enzymes don’t work properly, substances build up and can cause diseases such as Fabry disease.
The role of alpha-GAL.
alpha-GAL breaks down and clears GL-3 buildup
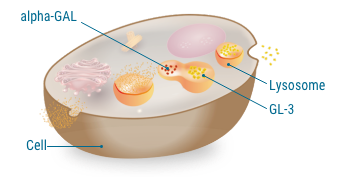
In people who don't have Fabry disease, lysosomes in the cells have alpha-GAL that can break down and clear GL-3.
GL-3 buildup with limited or no alpha-GAL
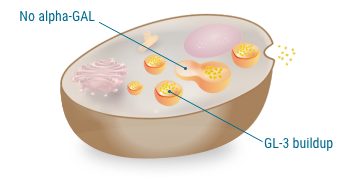
In people with Fabry disease, GL-3 builds up in lysosomes, so cells don't function as usual.
A lysosome is the digestive system of the cell, where fats and other substances are broken down.
Fabry disease and families.
Fabry disease affects people of all ages and ethnic backgrounds. It is inherited, which means that parents with Fabry disease can pass it down to their children.
Fabry disease runs in families, and when one person is diagnosed, an average of 5 additional relatives may also be affected.
If someone in your family has Fabry disease, it’s important to discuss testing options with your healthcare providers.
IMPACT OF DISEASE PROGRESSION
Some of the ways Fabry disease can affect you:
In people with Fabry disease, GL-3 starts to build up before birth and continues building up throughout life. Although everyone has GL-3, too much of it can cause damage to the body. Fabry disease affects women, men, and children differently. Even within the same family, people with Fabry disease may experience different symptoms including:
Impaired kidney function
- Protein in urine
- Kidney failure
Stomach disorders
- Diarrhea
- Constipation
- Stomach cramping
Strokes and ministrokes
Additional complications
- Hearing loss or ringing in the ears
- Whorling pattern in the eyes (corneal whorling)
- Headaches, lightheadedness, vertigo
- Breathing problems
Heart problems
- Chest pain
- Heart disease
- Enlarged heart
- Irregular heartbeat
Skin and nerve conditions
- Reddish or purple spots on skin
- Nerve pain in hands or feet
- Reduced ability to sweat
- Sensitivity to hot and cold temperatures
Long-term effects of Fabry disease.
As a result of GL-3 buildup, people with Fabry disease are at risk for problems that may become life threatening, such as kidney disease, heart problems, and early stroke. Some symptoms of Fabry disease may become worse over time without your knowledge.
UNDIAGNOSED AND UNMANAGED, FABRY DISEASE CAN REDUCE LIFE EXPECTANCY IN PATIENTS WITH CLASSIC DISEASE BY APPROXIMATELY:

Put your health first! It’s important to track your symptoms and see your doctor regularly.
IMPORTANCE OF MONITORING
Monitoring your health.
Managing and monitoring Fabry disease are important for maintaining your health. There are some symptoms that you can see and feel, which you should let your doctor know about. Some of these symptoms may include:
- Pain in the hands, feet, or stomach
- Inability to sweat
- Diarrhea
- Constipation
- Temperature sensitivities
- Reddish or purple spots on skin
- Heart palpitations
- Chest pain
Other symptoms can progress “silently” even if you don’t feel sick. Medical assessments are needed to monitor these symptoms, especially since they can affect the kidney, heart, and brain.
TESTS TO MONITOR FABRY DISEASE
Your doctor can order certain diagnostic tests and labs to measure the function of your kidneys, heart, and brain to understand the effects of Fabry disease on your body. Tests can be performed as often as every 6 months or as infrequently as every 3 years, depending on your age, clinical presentation, and/or other factors. More frequent testing may be needed if you experience new or more severe symptoms, or when you start or change your treatment plan.
HERE ARE SOME COMMON TESTS USED TO MONITOR FABRY DISEASE:
Glomerular filtration rate (GFR)
Measures level of kidney function and determines stage of kidney disease.
Tests for albuminuria and proteinuria
Measures excess protein in the urine. These tests typically indicate whether the kidneys are functioning normally.
Cranial or cardiac magnetic resonance imaging (MRI)
Measures tissue damage and detects a variety of conditions in the brain or heart.
Electrocardiogram (EKG/ECG)
Measures electrical activity in the heart.
Echocardiogram (Echo)
Measures thickness and assesses the function and structure of the heart.
We’re here to help! Ask your Patient Education Liaison about the schedule of assessments for Fabry disease. They can give you the tips and tools you need to monitor your symptoms.
TREATMENT GUIDELINES
Guiding treatment decisions.
Guidelines are a tool for physicians to consider when making treatment recommendations for their patients. People who have been diagnosed should receive regular check-ups, even if they are not experiencing symptoms or not currently on treatment. If you have questions about the guidelines, talk to your doctor.
The following guidelines have been developed by an international panel of Fabry experts for the treatment of Fabry disease. Your doctor will make specific testing and treatment decisions based upon your individual health considerations.
Enzyme replacement therapy (ERT) should be considered in symptomatic males and females at time of diagnosis.
- In published guidelines, Fabry disease experts recommend that ERT should be considered in symptomatic males and females at any age.*
ERT should be considered in asymptomatic males and females:
- Males with “classic” gene variants starting at ages 8 – 10.
- Males with “non-classic” gene variants and asymptomatic females:
- Should be monitored for the development of symptoms that warrant treatment with ERT: Problems in the kidneys, heart, or brain, as well as pain, gastrointestinal distress, difficulty sweating or exercise intolerance.
*Fabrazyme has not been studied in patients under the age of 2.
If you or any of your family members experience any of the above symptoms, talk to your doctor about your treatment goals.
Learn more about Fabry disease and treatment with Fabrazyme.
Download the Fabrazyme Patient Brochure (English)
See the clinical evidence
The safety of Fabrazyme has been assessed in 4 clinical trials involving 162 patients with over 473 patient-years of experience.
IMPORTANT SAFETY INFORMATION
WARNING: SEVERE ALLERGIC REACTIONS
Patients treated with enzyme replacement therapies have experienced allergic reactions, including severe or life-threatening reactions (known as anaphylaxis). Anaphylaxis has occurred during the early course and after repeated treatment with enzyme replacement therapy.
Your healthcare professional should initiate Fabrazyme in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment. Seek immediate medical care should symptoms occur.
Fabrazyme can cause serious side effects, including:
Severe Allergic Reactions Including Anaphylaxis
Approximately 1% of patients who received Fabrazyme experienced a severe allergic or anaphylactic reaction during their infusion. Some of these reactions were life-threatening, and included:
- Swelling of the face, mouth and throat, narrowing of breathing airways, low blood pressure, hives, difficulty swallowing, rash, trouble breathing, flushing, chest discomfort, itching and nasal congestion.
- Tell your healthcare professional if you experience any of these symptoms.
- Your healthcare professional may give you medicines before you receive Fabrazyme to help manage these reactions.
In clinical studies, some patients developed IgE antibodies or a reaction to an allergy skin test specific to Fabrazyme. IgE antibodies can sometimes be produced by the body’s immune system during an allergic reaction. Your healthcare professional may test you for IgE antibodies if you experience a suspected allergic reaction to help assess the risks and benefits of continuing treatment.
Infusion-Associated Reactions
In clinical studies, 59% of patients experienced infusion-associated reactions (IARs) during Fabrazyme administration, some of which were severe. IARs are defined as those occurring on the same day as your infusion. IARs occurred more frequently in patients who were positive for anti-Fabrazyme antibodies than those who did not have anti-Fabrazyme antibodies.
- You may receive medicines to help prevent IARs. IARs have happened in some patients even after taking these medications before their infusions.
- If an IAR occurs, tell your healthcare professional, who may slow the infusion rate, stop the infusion, and/or provide appropriate medical treatment as needed.
- People with advanced Fabry disease may have heart problems, which could put them at a higher risk for severe complications from IARs. Tell the healthcare professional for your infusions if you have known heart problems.
Common Side Effects
Side effects reported in 20% or more of Fabrazyme treated patients in clinical studies compared to placebo were upper respiratory tract infection, chills, fever, headache, cough, burning and/or tingling sensation, fatigue, swelling in the legs, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
IMPORTANT SAFETY INFORMATION
Show moreWARNING: SEVERE ALLERGIC REACTIONS
Patients treated with enzyme replacement therapies have experienced allergic reactions, including severe or life-threatening reactions (known as anaphylaxis). Anaphylaxis has occurred during the early course and after repeated treatment with enzyme replacement therapy.
Your healthcare professional should initiate Fabrazyme in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment. Seek immediate medical care should symptoms occur.
Fabrazyme can cause serious side effects, including:
Severe Allergic Reactions Including Anaphylaxis
Approximately 1% of patients who received Fabrazyme experienced a severe allergic or anaphylactic reaction during their infusion. Some of these reactions were life-threatening, and included:
- Swelling of the face, mouth and throat, narrowing of breathing airways, low blood pressure, hives, difficulty swallowing, rash, trouble breathing, flushing, chest discomfort, itching and nasal congestion.
- Tell your healthcare professional if you experience any of these symptoms.
- Your healthcare professional may give you medicines before you receive Fabrazyme to help manage these reactions.
In clinical studies, some patients developed IgE antibodies or a reaction to an allergy skin test specific to Fabrazyme. IgE antibodies can sometimes be produced by the body’s immune system during an allergic reaction. Your healthcare professional may test you for IgE antibodies if you experience a suspected allergic reaction to help assess the risks and benefits of continuing treatment.
Infusion-Associated Reactions
In clinical studies, 59% of patients experienced infusion-associated reactions (IARs) during Fabrazyme administration, some of which were severe. IARs are defined as those occurring on the same day as your infusion. IARs occurred more frequently in patients who were positive for anti-Fabrazyme antibodies than those who did not have anti-Fabrazyme antibodies.
- You may receive medicines to help prevent IARs. IARs have happened in some patients even after taking these medications before their infusions.
- If an IAR occurs, tell your healthcare professional, who may slow the infusion rate, stop the infusion, and/or provide appropriate medical treatment as needed.
- People with advanced Fabry disease may have heart problems, which could put them at a higher risk for severe complications from IARs. Tell the healthcare professional for your infusions if you have known heart problems.
Common Side Effects
Side effects reported in 20% or more of Fabrazyme treated patients in clinical studies compared to placebo were upper respiratory tract infection, chills, fever, headache, cough, burning and/or tingling sensation, fatigue, swelling in the legs, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
INDICATION AND USAGE
Fabrazyme® is used to treat adults and children 2 years of age and older with confirmed Fabry disease.

