PUT WHAT MATTERS FIRST WITH FABRAZYME®
Fabrazyme is an enzyme replacement therapy (ERT) that supplies the enzyme that’s missing or deficient in Fabry disease. The fully functional enzyme helps clear GL-3 buildup in your cells.



HOW FABRAZYME WORKS
Fabrazyme helps to clear GL-3 buildup by replacing the missing enzyme in Fabry disease.
Watch this video to learn how Fabrazyme works inside the body.

FABRAZYME CAN BE USED FOR ALL PATIENTS 2 YEARS AND OLDER REGARDLESS OF GENETIC VARIANT, GENDER, OR DISEASE SEVERITY.

Unsure about a medical term? Find definitions here.

Learn more about Fabry disease and treatment with Fabrazyme.
Download the Fabrazyme Patient Brochure (English)
HOW FABRAZYME CAN HELP
Fabrazyme has been proven to clear GL-3 buildup in certain cells of the body.
Study 1 design: This study included 58 Fabry patients ages 16-61. Patients in this study received either Fabrazyme or placebo every 2 weeks for 5 months. Patients received a score of 0 to 3 based on the amount of GL-3 in their cells. After treatment with Fabrazyme, most people received a score of 0, meaning the GL-3 in their cells was nearly none or trace amounts.
Study 1 extension design: All 58 patients who completed Study 1 were treated with Fabrazyme every 2 weeks in a 6-month open-label extension study, in which patients and researchers both knew which treatment the patient was receiving during the study.
In Study 1, after 5 months of treatment with Fabrazyme, most patients achieved GL-3 clearance resulting in trace, or nearly none, GL-3 inclusions in certain cells of their: Kidney: 69% (20/29) of Fabrazyme patients compared with 0% (0/29) of placebo patients; Heart: 72% (21/29) of Fabrazyme patients compared with 3% (1/29) of placebo patients; Skin: 100% (29/29) of Fabrazyme patients compared with 3% (1/29) of placebo patients.

In this extension study, similar long-term GL-3 clearance was observed at 4.5 years in certain cells of the kidney (8/8) and heart (6/8).
Patients who regularly received Fabrazyme for up to 5 years maintained normal GL-3 levels in their blood.
A smaller percentage of individuals treated with Fabrazyme experienced a clinically significant event.*
A clinically significant event is defined as the first instance in any of the four categories below, which occurred after the study started.
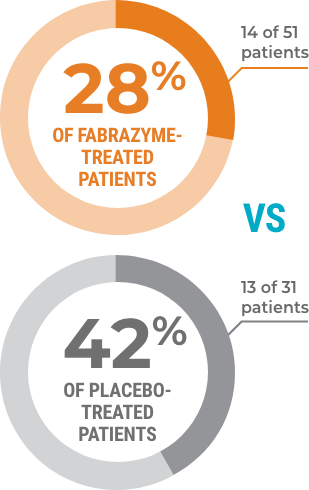
A SMALLER PERCENTAGE OF PEOPLE HAD HEART, KIDNEY, STROKE EVENTS, OR DEATH.
(HR=0.57, 95% CI: 0.27, 1.22, P=0.14)
Kidneys
Heart
Stroke events
Loss of life
Study design: A randomized, double-blind, placebo-controlled, multinational, multicenter study of 82 patients (72 males and 10 females) with Fabry disease. Patients were 20 to 72 years of age with a median age of 45 years at baseline, a median age of 36 years at Fabry disease diagnosis, and at a median of 10 years at symptom onset.
*Renal, cardiac, or stroke events or death.

FABRAZYME IS THE ONLY ERT INDICATED FOR PATIENTS 2 YEARS OF AGE AND OLDER THAT HAS PROVEN LONG-TERM EFFICACY AND SAFETY.
In an observational study of 24 pediatric patients aged 2-7 years, normalization of plasma GL-3 was observed.
In children 8 years and older, Fabrazyme cleared GL-3 in certain cells of their skin and blood.
Study overview: 16 pediatric patients with Fabry disease, aged 8-16 years, were evaluated in an open-label, uncontrolled study.




The most common adverse reactions (>20%) in patients aged 8-16 were headache, abdominal pain, sore throat, fever, nausea, vomiting, nasal inflammation, diarrhea, joint pain, and dizziness.

THE OVERALL SAFETY PROFILE WAS SIMILAR BETWEEN THE PEDIATRIC AND THE ADULT POPULATION.
The rate of kidney function decline was studied in Fabrazyme-treated individuals.
One of the ways your doctor measures kidney function is to monitor your eGFR—a calculation made using a blood test to determine how your kidneys are working. Declining eGFR may indicate a decline in kidney function.
estimated difference in the rate of kidney function decline
The rate of renal function decline was assessed in Fabry disease patients aged ≥16 years. The mean slope of eGFR* was -1.5 mL/min/1.73m2/year in the Fabrazyme-treated group and -3.2 mL/min/1.73m2/year in the untreated group. The mean difference in eGFR between the two groups was 1.7 mL/min/1.73m2/year.
Study design: In a long-term observational study, the rate of decline in renal function (eGFR slope) was assessed in 122 patients with Fabry disease aged 16 years and older treated with Fabrazyme and matched to a historical cohort of untreated patients.
*eGFR slope is a measurement of kidney function over time.
SAFETY PROFILE
The safety profile of Fabrazyme has been well established.
THE SAFETY OF FABRAZYME HAS BEEN ASSESSED IN 4 CLINICAL STUDIES INVOLVING 162 PATIENTS WITH OVER 473 PATIENT-YEARS OF EXPERIENCE
In clinical trials, common side effects that occurred in 20% or more of people treated with Fabrazyme, and in more than 2.5% of people who received placebo (control group), include:
|
Side effect |
Fabrazyme (n=80) |
Placebo (n=60) |
|
Upper respiratory tract infection |
53% |
42% |
|
Chills |
49% |
13% |
|
Fever (pyrexia) |
39% |
22% |
|
Headache |
39% |
28% |
|
Cough |
33% |
25% |
|
Burning and/or tingling sensation in hands and feet (paresthesia) |
31% |
18% |
|
Fatigue |
24% |
17% |
|
Swelling in the limbs (peripheral edema) |
21% |
7% |
|
Dizziness |
21% |
8% |
|
Rash |
20% |
10% |
n=number of patients.
Read below for more information about what to expect from your infusions.
Serious side effects
- Life-threatening severe anaphylactic (allergic) or severe hypersensitivity reactions have been seen in patients during Fabrazyme infusions
- Approximately 1% of patients who have received Fabrazyme either during a clinical study or after Fabrazyme was approved have experienced anaphylactic (allergic) or severe hypersensitivity reactions during their infusion
- In clinical studies, 59% of patients experienced infusion-associated reactions during Fabrazyme treatment, some of which were severe
- During the clinical trials, infusion-associated reactions occurred more frequently in patients who were positive for anti-Fabrazyme antibodies than in patients who did not have anti-Fabrazyme antibodies
- People with advanced Fabry disease may have heart problems, which may put them at a higher risk for severe complications from infusion-associated reactions. These patients should be watched closely during their infusion if the decision is made to give them Fabrazyme
Talk to your doctor about any side effects you experience when taking Fabrazyme.
STARTING FABRAZYME
Treatment with Fabrazyme.
Fabrazyme can be given in a number of treatment settings, including:
DOCTOR’S OFFICE
INFUSION CENTERS
HOME
HOSPITAL
Talk to your doctor about which treatment setting is right for you.
Make your treatments a priority.
Fabrazyme is an infusion that’s given to help prevent the buildup of GL-3. Fabrazyme keeps helping to clear GL-3 as long as you continue treatment.
Follow the Fabrazyme treatment plan prescribed by your doctor, even if you’re not experiencing symptoms.
WHAT TO EXPECT THROUGHOUT TREATMENT
What to expect before, during, and after treatment.
Your first infusion of Fabrazyme will be given in a doctor’s office, hospital, or infusion center. After several infusions, your doctor may give you the option of receiving Fabrazyme in your home.
To better incorporate infusions into your life, you may be able to request a specific day and/or time for your treatment. It is also a good idea to arrange for transportation to and from the infusion site—especially on your first visit.
Before you have your infusion, you may have an assessment by a healthcare professional, which can include asking you how you are currently feeling, measuring your weight, your heart rate, blood pressure, and temperature. Next, you may be given anti-fever (such as acetaminophen or ibuprofen) and antihistamine medications to help prevent or reduce infusion-associated reactions.
How Fabrazyme is delivered
Following the assessment, the healthcare professional will start your IV, either through your port or through a vein in your arm. This is how Fabrazyme will get into your body during the infusion.
Talk to your doctors about your potential for infusion-related side effects, and how they may be managed. Please see Important Safety Information for Infusion-Associated Reactions.
Be sure to follow the treatment schedule your doctor recommends.
Sometimes life’s challenges—being too busy, traveling away from home—can get in the way of receiving your Fabrazyme infusion. But it is important to follow the infusion schedule your doctor prescribes.
Fabrazyme keeps working when you keep taking it
Regular infusions of Fabrazyme every two weeks help ensure that the body has an ongoing supply of fully functional enzyme to clear GL-3 in certain cells of the kidney, heart, and skin. Even if you don’t notice any symptoms, it’s important to continue to take Fabrazyme as directed, to help ensure that GL-3 doesn’t start to build up again.
Finding Fabry terms confusing?
We’ve got you covered. Refer to our glossary of terms to help with your discussions about Fabry.
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: SEVERE ALLERGIC REACTIONS
Patients treated with enzyme replacement therapies have experienced allergic reactions, including severe or life-threatening reactions (known as anaphylaxis). Anaphylaxis has occurred during the early course and after repeated treatment with enzyme replacement therapy.
Your healthcare professional should initiate Fabrazyme in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment. Seek immediate medical care should symptoms occur.
Fabrazyme can cause serious side effects, including:
Severe Allergic Reactions Including Anaphylaxis
Approximately 1% of patients who received Fabrazyme experienced a severe allergic or anaphylactic reaction during their infusion. Some of these reactions were life-threatening, and included:
- Swelling of the face, mouth and throat, narrowing of breathing airways, low blood pressure, hives, difficulty swallowing, rash, trouble breathing, flushing, chest discomfort, itching and nasal congestion.
- Tell your healthcare professional if you experience any of these symptoms.
- Your healthcare professional may give you medicines before you receive Fabrazyme to help manage these reactions.
In clinical studies, some patients developed IgE antibodies or a reaction to an allergy skin test specific to Fabrazyme. IgE antibodies can sometimes be produced by the body’s immune system during an allergic reaction. Your healthcare professional may test you for IgE antibodies if you experience a suspected allergic reaction to help assess the risks and benefits of continuing treatment.
Infusion-Associated Reactions
In clinical studies, 59% of patients experienced infusion-associated reactions (IARs) during Fabrazyme administration, some of which were severe. IARs are defined as those occurring on the same day as your infusion. IARs occurred more frequently in patients who were positive for anti-Fabrazyme antibodies than those who did not have anti-Fabrazyme antibodies.
- You may receive medicines to help prevent IARs. IARs have happened in some patients even after taking these medications before their infusions.
- If an IAR occurs, tell your healthcare professional, who may slow the infusion rate, stop the infusion, and/or provide appropriate medical treatment as needed.
- People with advanced Fabry disease may have heart problems, which could put them at a higher risk for severe complications from IARs. Tell the healthcare professional for your infusions if you have known heart problems.
Common Side Effects
Side effects reported in 20% or more of Fabrazyme treated patients in clinical studies compared to placebo were upper respiratory tract infection, chills, fever, headache, cough, burning and/or tingling sensation, fatigue, swelling in the legs, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
IMPORTANT SAFETY INFORMATION
Show moreIMPORTANT SAFETY INFORMATION
WARNING: SEVERE ALLERGIC REACTIONS
Patients treated with enzyme replacement therapies have experienced allergic reactions, including severe or life-threatening reactions (known as anaphylaxis). Anaphylaxis has occurred during the early course and after repeated treatment with enzyme replacement therapy.
Your healthcare professional should initiate Fabrazyme in a healthcare setting with appropriate medical monitoring and support measures. If a severe allergic or anaphylactic reaction occurs, your healthcare professional will immediately stop the infusion and provide appropriate medical treatment. Seek immediate medical care should symptoms occur.
Fabrazyme can cause serious side effects, including:
Severe Allergic Reactions Including Anaphylaxis
Approximately 1% of patients who received Fabrazyme experienced a severe allergic or anaphylactic reaction during their infusion. Some of these reactions were life-threatening, and included:
- Swelling of the face, mouth and throat, narrowing of breathing airways, low blood pressure, hives, difficulty swallowing, rash, trouble breathing, flushing, chest discomfort, itching and nasal congestion.
- Tell your healthcare professional if you experience any of these symptoms.
- Your healthcare professional may give you medicines before you receive Fabrazyme to help manage these reactions.
In clinical studies, some patients developed IgE antibodies or a reaction to an allergy skin test specific to Fabrazyme. IgE antibodies can sometimes be produced by the body’s immune system during an allergic reaction. Your healthcare professional may test you for IgE antibodies if you experience a suspected allergic reaction to help assess the risks and benefits of continuing treatment.
Infusion-Associated Reactions
In clinical studies, 59% of patients experienced infusion-associated reactions (IARs) during Fabrazyme administration, some of which were severe. IARs are defined as those occurring on the same day as your infusion. IARs occurred more frequently in patients who were positive for anti-Fabrazyme antibodies than those who did not have anti-Fabrazyme antibodies.
- You may receive medicines to help prevent IARs. IARs have happened in some patients even after taking these medications before their infusions.
- If an IAR occurs, tell your healthcare professional, who may slow the infusion rate, stop the infusion, and/or provide appropriate medical treatment as needed.
- People with advanced Fabry disease may have heart problems, which could put them at a higher risk for severe complications from IARs. Tell the healthcare professional for your infusions if you have known heart problems.
Common Side Effects
Side effects reported in 20% or more of Fabrazyme treated patients in clinical studies compared to placebo were upper respiratory tract infection, chills, fever, headache, cough, burning and/or tingling sensation, fatigue, swelling in the legs, dizziness, and rash.
Please see full Prescribing Information, including Boxed WARNING
INDICATION AND USAGE
Fabrazyme® is used to treat adults and children 2 years of age and older with confirmed Fabry disease.

